Page 71 - OHKF_Biotech_EN
P. 71
drugs and medical devices to go through the conventional
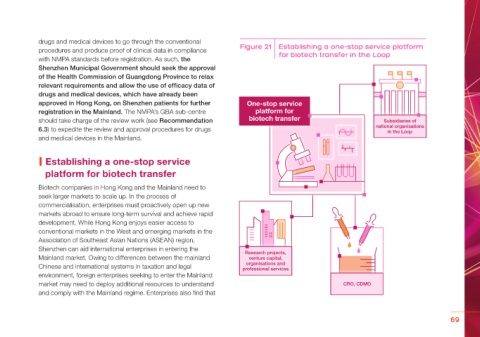
procedures and produce proof of clinical data in compliance Figure 21 Establishing a one-stop service platform
for biotech transfer in the Loop
with NMPA standards before registration. As such, the
Shenzhen Municipal Government should seek the approval
of the Health Commission of Guangdong Province to relax
relevant requirements and allow the use of efficacy data of
drugs and medical devices, which have already been
approved in Hong Kong, on Shenzhen patients for further One-stop service
registration in the Mainland. The NMPA’s GBA sub-centre platform for
should take charge of the review work (see Recommendation biotech transfer Subsidiaries of
6.3) to expedite the review and approval procedures for drugs national organisations
in the Loop
and medical devices in the Mainland.
| Establishing a one-stop service
platform for biotech transfer
Biotech companies in Hong Kong and the Mainland need to
seek larger markets to scale up. In the process of
commercialisation, enterprises must proactively open up new
markets abroad to ensure long-term survival and achieve rapid
development. While Hong Kong enjoys easier access to
conventional markets in the West and emerging markets in the
Association of Southeast Asian Nations (ASEAN) region,
Shenzhen can aid international enterprises in entering the Research projects,
Mainland market. Owing to differences between the mainland venture capital,
organisations and
Chinese and international systems in taxation and legal professional services
environment, foreign enterprises seeking to enter the Mainland
market may need to deploy additional resources to understand CRO, CDMO
and comply with the Mainland regime. Enterprises also find that
69