Page 66 - OHKF_Biotech_EN
P. 66
5
•
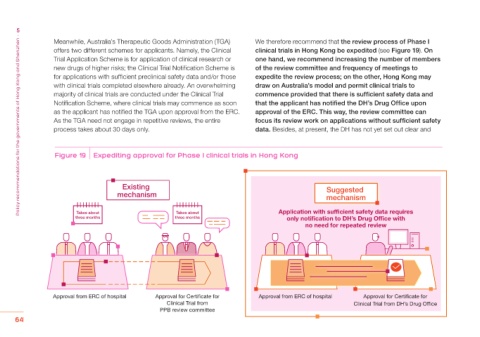
Meanwhile, Australia’s Therapeutic Goods Administration (TGA) We therefore recommend that the review process of Phase I
Policy recommendations for the governments of Hong Kong and Shenzhen
offers two different schemes for applicants. Namely, the Clinical clinical trials in Hong Kong be expedited (see Figure 19). On
Trial Application Scheme is for application of clinical research or one hand, we recommend increasing the number of members
new drugs of higher risks; the Clinical Trial Notification Scheme is of the review committee and frequency of meetings to
for applications with sufficient preclinical safety data and/or those expedite the review process; on the other, Hong Kong may
with clinical trials completed elsewhere already. An overwhelming draw on Australia’s model and permit clinical trials to
majority of clinical trials are conducted under the Clinical Trial commence provided that there is sufficient safety data and
Notification Scheme, where clinical trials may commence as soon that the applicant has notified the DH’s Drug Office upon
as the applicant has notified the TGA upon approval from the ERC. approval of the ERC. This way, the review committee can
As the TGA need not engage in repetitive reviews, the entire focus its review work on applications without sufficient safety
process takes about 30 days only. data. Besides, at present, the DH has not yet set out clear and
Figure 19 Expediting approval for Phase I clinical trials in Hong Kong
Existing Suggested
mechanism mechanism
Takes about Takes about Application with suf cient safety data requires
three months three months only noti cation to DH’s Drug Of ce with
no need for repeated review
Approval from ERC of hospital Approval for Certi cate for Approval from ERC of hospital Approval for Certi cate for
Clinical Trial from Clinical Trial from DH’s Drug Of ce
PPB review committee
64