Page 63 - OHKF_Biotech_EN
P. 63
preclinical trials, regulators will only accept data from laboratories of the world. While there are 12 and 11 NMPA GLP-accredited
57
that conform to the their standard guidelines (i.e., GLP, Good laboratories in Shanghai and Beijing respectively, Hong Kong
Laboratory Practice). Currently, Hong Kong and Shenzhen have no and Shenzhen currently have none that has received accreditation,
GLP-accredited laboratories recognised by major drug regulators and each city has only one meeting the relevant standards (but
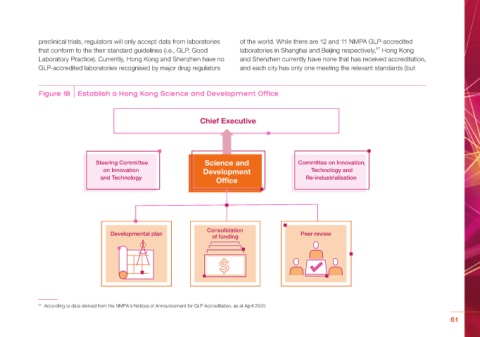
Figure 18 Establish a Hong Kong Science and Development Office
Chief Executive
Steering Committee Science and Committee on Innovation,
on Innovation Development Technology and
and Technology Of ce Re-industrialisation
Consolidation
Developmental plan of funding Peer review
57 According to data derived from the NMPA’s Notices of Announcement for GLP Accreditation, as at April 2020.
61