Page 36 - OHKF_Biotech_EN
P. 36
4
•
Visions for the HK-SZ Collaborative Ecosystem in Biotech
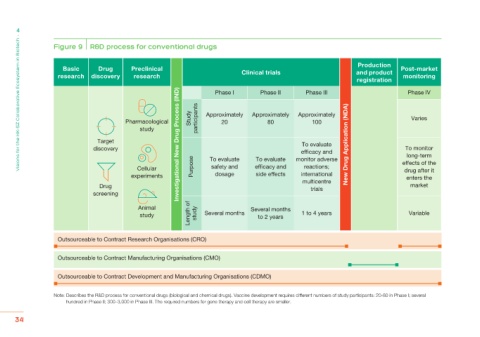
Figure 9 R&D process for conventional drugs
Production
Basic Drug Preclinical Clinical trials and product Post-market
research discovery research monitoring
registration Phase IV
Phase I
Phase II
Phase III
Approximately Approximately Approximately
Pharmacological Study participants 20 80 100 Varies
study
Target Investigational New Drug Process (IND) To evaluate New Drug Application (NDA)
discovery efficacy and To monitor
long-term
To evaluate To evaluate monitor adverse effects of the
Cellular Purpose safety and efficacy and reactions; drug after it
experiments dosage side effects international enters the
Drug multicentre market
trials
screening
Animal Several months
study Length of study Several months to 2 years 1 to 4 years Variable
Outsourceable to Contract Research Organisations (CRO)
Outsourceable to Contract Manufacturing Organisations (CMO)
Outsourceable to Contract Development and Manufacturing Organisations (CDMO)
Note: Describes the R&D process for conventional drugs (biological and chemical drugs). Vaccine development requires different numbers of study participants: 20-80 in Phase I; several
hundred in Phase II; 300–3,000 in Phase III. The required numbers for gene therapy and cell therapy are smaller.
34