Page 38 - OHKF_Biotech_EN
P. 38
4
•
Visions for the HK-SZ Collaborative Ecosystem in Biotech
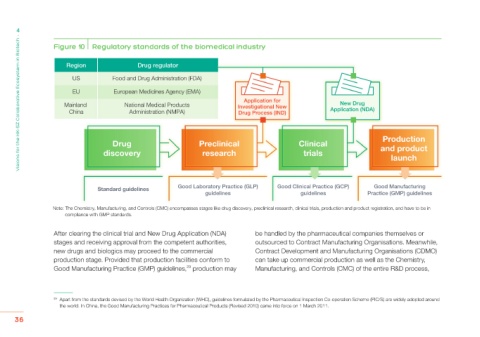
Figure 10 Regulatory standards of the biomedical industry
Region Drug regulator
US Food and Drug Administration (FDA)
EU European Medicines Agency (EMA)
Application for
Mainland National Medical Products Investigational New New Drug
China Administration (NMPA) Drug Process (IND) Application (NDA)
Production
Drug Preclinical Clinical
discovery research trials and product
launch
Standard guidelines Good Laboratory Practice (GLP) Good Clinical Practice (GCP) Good Manufacturing
guidelines guidelines Practice (GMP) guidelines
Note: The Chemistry, Manufacturing, and Controls (CMC) encompasses stages like drug discovery, preclinical research, clinical trials, production and product registration, and have to be in
compliance with GMP standards.
After clearing the clinical trial and New Drug Application (NDA) be handled by the pharmaceutical companies themselves or
stages and receiving approval from the competent authorities, outsourced to Contract Manufacturing Organisations. Meanwhile,
new drugs and biologics may proceed to the commercial Contract Development and Manufacturing Organisations (CDMO)
production stage. Provided that production facilities conform to can take up commercial production as well as the Chemistry,
29
Good Manufacturing Practice (GMP) guidelines, production may Manufacturing, and Controls (CMC) of the entire R&D process,
29 Apart from the standards devised by the World Health Organization (WHO), guidelines formulated by the Pharmaceutical Inspection Co-operation Scheme (PIC/S) are widely adopted around
the world. In China, the Good Manufacturing Practices for Pharmaceutical Products (Revised 2010) came into force on 1 March 2011.
36