Page 40 - OHKF_Biotech_EN
P. 40
4
•
33
Hong Kong, Shenzhen and the GBA (see Recommendations
Visions for the HK-SZ Collaborative Ecosystem in Biotech
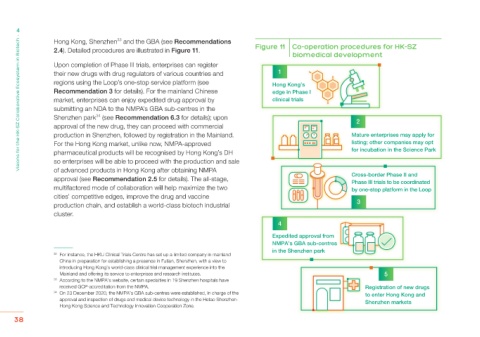
2.4). Detailed procedures are illustrated in Figure 11. Figure 11 Co-operation procedures for HK-SZ
biomedical development
Upon completion of Phase III trials, enterprises can register
their new drugs with drug regulators of various countries and 1
regions using the Loop’s one-stop service platform (see Hong Kong’s
Recommendation 3 for details). For the mainland Chinese edge in Phase I
market, enterprises can enjoy expedited drug approval by clinical trials
submitting an NDA to the NMPA’s GBA sub-centres in the
34
Shenzhen park (see Recommendation 6.3 for details); upon 2
approval of the new drug, they can proceed with commercial
production in Shenzhen, followed by registration in the Mainland. Mature enterprises may apply for
For the Hong Kong market, unlike now, NMPA-approved listing; other companies may opt
pharmaceutical products will be recognised by Hong Kong’s DH for incubation in the Science Park
so enterprises will be able to proceed with the production and sale
of advanced products in Hong Kong after obtaining NMPA
approval (see Recommendation 2.5 for details). The all-stage, Cross-border Phase II and
Phase III trials to be coordinated
multifactored mode of collaboration will help maximize the two by one-stop platform in the Loop
cities’ competitive edges, improve the drug and vaccine
production chain, and establish a world-class biotech industrial 3
cluster.
4
Expedited approval from
NMPA’s GBA sub-centres
in the Shenzhen park
32 For instance, the HKU Clinical Trials Centre has set up a limited company in mainland
China in preparation for establishing a presence in Futian, Shenzhen, with a view to
introducing Hong Kong’s world-class clinical trial management experience into the
Mainland and offering its service to enterprises and research institutes. 5
33 According to the NMPA’s website, certain specialties in 19 Shenzhen hospitals have
received GCP-accreditation from the NMPA. Registration of new drugs
34 On 23 December 2020, the NMPA’s GBA sub-centres were established, in charge of the to enter Hong Kong and
approval and inspection of drugs and medical device technology in the Hetao Shenzhen- Shenzhen markets
Hong Kong Science and Technology Innovation Cooperation Zone.
38