Page 92 - OHKF_Gerontech_report_en
P. 92
“Don’t know”
Gaps Significantly Slightly No change Slightly Significantly or Final score
worsened worsened improved improved “no opinion”
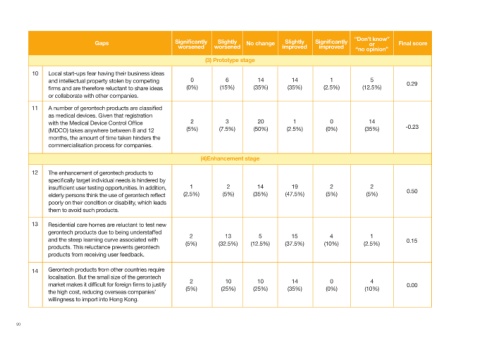
(3) Prototype stage
10 Local start-ups fear having their business ideas
and intellectual property stolen by competing 0 6 14 14 1 5 0.29
firms and are therefore reluctant to share ideas (0%) (15%) (35%) (35%) (2.5%) (12.5%)
or collaborate with other companies.
11 A number of gerontech products are classified
as medical devices. Given that registration
with the Medical Device Control Office 2 3 20 1 0 14
(MDCO) takes anywhere between 8 and 12 (5%) (7.5%) (50%) (2.5%) (0%) (35%) -0.23
months, the amount of time taken hinders the
commercialisation process for companies.
(4)Enhancement stage
12 The enhancement of gerontech products to
specifically target individual needs is hindered by
insufficient user testing opportunities. In addition, 1 2 14 19 2 2 0.50
elderly persons think the use of gerontech reflect (2.5%) (5%) (35%) (47.5%) (5%) (5%)
poorly on their condition or disability, which leads
them to avoid such products.
13 Residential care homes are reluctant to test new
gerontech products due to being understaffed
2 13 5 15 4 1
and the steep learning curve associated with 0.15
(5%) (32.5%) (12.5%) (37.5%) (10%) (2.5%)
products. This reluctance prevents gerontech
products from receiving user feedback.
14 Gerontech products from other countries require
localisation. But the small size of the gerontech
2 10 10 14 0 4
market makes it difficult for foreign firms to justify 0.00
(5%) (25%) (25%) (35%) (0%) (10%)
the high cost, reducing overseas companies’
willingness to import into Hong Kong.
90